Articles from Epitel, Inc.

Epitel, a leader in AI-driven brain health solutions, today announced that the U.S. Food and Drug Administration (FDA) has granted another 510(k) Clearance for the REMI™ Wireless EEG System, expanding the Indications for Use of their revolutionary wireless EEG system in infants and children one year of age and older. This designation underscores the potential of REMI to revolutionize neurological monitoring in one of the most vulnerable patient populations.
By Epitel, Inc. · Via Business Wire · March 31, 2025

Epitel, Inc., a leader in AI technologies for patient-focused brain health solutions, is pleased to announce that Steve Pacelli has been appointed as the company's new Chief Executive Officer. Pacelli, a seasoned executive with a track record of success in the medical technology industry, brings a wealth of experience and leadership to Epitel as it further drives its mission to transform the lives of individuals affected by seizures and other neurological conditions. As the executive team expands, Mark Lehmkuhle, the company’s current CEO and founder, will transition to the role of Chief Technology Officer.
By Epitel, Inc. · Via Business Wire · February 13, 2025

Epitel, a leader in AI for patient-focused brain health technologies, announced today it received U.S. Food and Drug Administration (FDA) 510(k) clearance for REMI Vigilenz™ AI For Bedside Notifications. REMI Vigilenz AI For Bedside Notifications conducts an automated analysis of EEG data collected by the REMI™ wireless wearable EEG System in near real-time. Notifications occur when the algorithm recognizes seizure characteristics within a section of EEG. These notifications provide information about the potential electrographic seizure, including seizure burden and corresponding confidence level.
By Epitel, Inc. · Via Business Wire · October 21, 2024
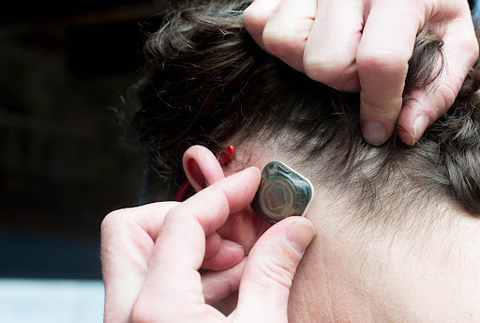
Epitel, Inc., a digital health company developing a wearable, wireless EEG monitoring platform for seizure detection, announced today the closing of a $12.5 million Series A financing for initial pilot commercialization and further development of its proprietary platform. Epitel received FDA clearance for its first product, a wireless and wearable EEG (brain wave monitor) sensor, and remote access software known as REMI® for use within hospital emergency rooms and critical care units. REMI first received clearance from the U.S. Food & Drug Administration in 2021.
By Epitel, Inc. · Via Business Wire · February 16, 2022
