Articles from Propanc Biopharma, Inc.

MELBOURNE, Australia, Nov. 04, 2025 (GLOBE NEWSWIRE) -- Propanc Biopharma, Inc. (Nasdaq: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing new treatments for patients suffering from recurrent and metastatic cancer, today announced a strategic initiative to pursue the acquisition of Digital Asset Treasury (DAT) companies that are currently trading below their Market Cap to Net Asset Value (MNAV).
By Propanc Biopharma, Inc. · Via GlobeNewswire · November 4, 2025

Initial Conversion Price of $5.00 Per Share Representing a 280% Premium Over the Company’s Recent Closing Price of $1.78
By Propanc Biopharma, Inc. · Via GlobeNewswire · October 15, 2025

Initial Conversion Price of $5.00 Per Share Representing a 280% Premium Over the Company’s Recent Closing Price of $1.78
By Propanc Biopharma, Inc. · Via GlobeNewswire · October 15, 2025

MELBOURNE, Australia, Oct. 07, 2025 (GLOBE NEWSWIRE) -- Propanc Biopharma, Inc. (Nasdaq: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company focused on developing treatments for recurring and metastatic cancer, announced its fiscal year-end update following the filing of its annual 10-K report with the Securities and Exchange Commission on September 29, 2025. The reporting period concluded on June 30, 2025.
By Propanc Biopharma, Inc. · Via GlobeNewswire · October 7, 2025

Fourth US Granted Patent Captures Claims for Future Clinical Dose of PRP
By Propanc Biopharma, Inc. · Via GlobeNewswire · September 17, 2025

Ethereum to Enhance Multi-Faceted Corporate Strategy of Cryptocurrency Exposure, Pharmaceutical Drug Development & Asset Acquisition
By Propanc Biopharma, Inc. · Via GlobeNewswire · September 2, 2025

MELBOURNE, Australia, Aug. 25, 2025 (GLOBE NEWSWIRE) -- Propanc Biopharma, Inc. (Nasdaq: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced a shareholder update including recent corporate developments and forecast for 2025/26. As a result of completing a recent initial public offering and up-listing to Nasdaq, the Company is entering a transformational stage as it prepares for the advancement of its lead asset, “PRP”, to enter a Phase 1B, First-In-Human (FIH) study in 30 – 40 advanced cancer patients suffering from malignant solid tumors designed to identify the maximum tolerated dose (MTD) in 2026.
By Propanc Biopharma, Inc. · Via GlobeNewswire · August 25, 2025

MELBOURNE, Australia, Aug. 19, 2025 (GLOBE NEWSWIRE) -- Propanc Biopharma, Inc. (Nasdaq: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that it closed an underwritten public offering of 1,000,000 shares of its common stock, par value $0.001 per share, at a price of $4.00 per share. The shares of common stock commenced trading on the Nasdaq Capital Market on August 15, 2025, under the ticker symbol, “PPCB”.
By Propanc Biopharma, Inc. · Via GlobeNewswire · August 19, 2025

Propanc common stock expected to begin trading on Nasdaq under the symbol PPCB
By Propanc Biopharma, Inc. · Via GlobeNewswire · August 14, 2025

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that two scientific, peer reviewed journal articles published by the Company and its research partners reached 10 citations and 4,500 reads, respectively, in August, 2024. “This shows unprecedented interest in our field from researchers and among the broader scientific community,” said Dr Kenyon, MD, MB, ChB, Propanc’s Chief Scientific Officer.
By Propanc Biopharma, Inc. · Via Business Wire · August 21, 2024

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that allowance for the Company’s “proenzyme composition” patent was received from the Canadian Intellectual Property Office (CIPO). The patent broadly captures both high dose and high ratio claims for future clinical doses of the company’s lead asset, PRP. This is the second Canadian patent either allowed or granted in this important North American jurisdiction. Currently, the Company’s intellectual property portfolio consists of 93 patents filed in major jurisdictions relating to the use of PRP against solid tumors.
By Propanc Biopharma, Inc. · Via Business Wire · August 14, 2024

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company’s lead asset, PRP, could solve the problem that impacts the response rate of immune checkpoint inhibitors treating PD-L1-High (Programmed Death-Ligand 1) solid tumors, such as lung cancer. Propanc’s Chief Scientific Officer and Co-Founder Dr Julian Kenyon, MD, MB, ChB, predicts that pretreatment of PD-L1-high solid tumors with PRP as a combinatorial approach could reverse the promotion of epithelial to mesenchymal transition (EMT) pathways induced by PD-L1, a fundamental process by which malignant cells promote tumor growth and metastasis. Tumor cells that undergo the EMT are motile and invasive, spreading and seeding new tumors, as well as become immortal, no longer dying off naturally. They are also non-dividing, which means they are resistant to standard treatment approaches.
By Propanc Biopharma, Inc. · Via Business Wire · July 17, 2024

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that a Certificate of Grant for the Company’s “proenzymes composition” patent was received from the Japanese Patent Office. The patent covers dosing regimen claims for the company’s lead asset, PRP, which is advancing to clinical development. This is the fourth Japanese patent either allowed or granted in this important jurisdiction. Also, a certificate of Grant was also received from the Intellectual Property (IP) Corporation of Malaysia for a “cancer treatment” using therapeutically effective amounts of two proenzymes, trypsinogen and chymotrypsinogen. This is the third granted patent in Malaysia. Currently, the Company’s intellectual property portfolio consists of 93 patents filed in major global jurisdictions relating to the use of PRP against solid tumors.
By Propanc Biopharma, Inc. · Via Business Wire · June 25, 2024

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that a certificate of grant for the Company’s “composition of proenzymes for cancer treatment,” patent was received from the European Patent Office. The patent covers lower dosage ratios of the two proenzymes (trypsinogen and chymotrypsinogen) contained in the PRP formulation. This is the fourth European patent granted and after validation in selected countries across Europe, will result in the Company’s IP portfolio growing to 94 patents filed in major global jurisdictions.
By Propanc Biopharma, Inc. · Via Business Wire · April 16, 2024

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that a certificate of grant for the Company’s foundation patent was received from the Canadian Intellectual Property Office. The foundation patent covers composition claims for the Company’s lead product candidate, PRP. In further news, the PRP dosing and method to treat cancer stem cells (CSCs) patents were validated in countries across Europe, resulting in the Company’s IP portfolio growing to 87 patents filed in major global jurisdictions.
By Propanc Biopharma, Inc. · Via Business Wire · January 16, 2024

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that a boutique advisory firm has been engaged to identify strategic investment opportunities primarily to fund the advancement of the Company’s lead product candidate, PRP, to the completion of the Company’s planned Phase I, First-In-Human study in advanced cancer patients suffering from solid tumors. Funds will also be utilized to advance the Company’s back up clinical candidate, Rec-PRP, a synthetic recombinant version of PRP, which will further enhance the potency and stability of the naturally derived proenzyme treatment. As part of the process, the opportunity to consider up-listing to a national US stock exchange will also be considered. The emerging boutique advisory firm currently has a $3B+ transaction history and access to a global network of investors the Company intends to fully utilize.
By Propanc Biopharma, Inc. · Via Business Wire · December 18, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company’s joint researcher, Mrs. Belén Toledo Cutillas, has commenced an internship at the Cancer Center Amsterdam – vUMC (University Medical Centers). In addition to Propanc Biopharma providing the financial resources to develop PRP, funding from two international grants has enabled Mrs. Toledo to join the Molecular Oncology Laboratory at the Cancer Center Amsterdam. Research by this team of scientists, led by Professor Elisa Giovanetti, focuses on chemotherapy resistance in pancreatic cancer.
By Propanc Biopharma, Inc. · Via Business Wire · December 13, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that PRP enhances the sensitivity of resistant pancreatic tumor cells to standard chemotherapy treatment and alters the tumor microenvironment by decreasing the fibrotic tissue and its malignancy (ability to spread into surrounding tissues). The results were reported by the Company’s joint researcher, Mrs. Belén Toledo Cutillas, MSc, at the laboratory of Professor Macarena Perán, PhD, University of Jaén, Granada, Spain.
By Propanc Biopharma, Inc. · Via Business Wire · November 7, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company will target the Peter Mac Cancer Center in Melbourne, Australia, as the site for the First-In-Human (FIH) study for PRP in patients with advanced solid tumors. Initial discussion held previously with the Clinical Investigator confirmed the remaining development activities to be conducted by the Company prior to filing the clinical trial application for the FIH study. This includes the finished drug product manufacture and validation of the bioanalytical method to analyze the pharmacokinetics (movement of drug within the body) of PRP during the study.
By Propanc Biopharma, Inc. · Via Business Wire · August 15, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company’s clinical candidate PRP targeting the pancreatic cancer market is forecast to reach $6.93 Billion by 2030, according to a report published by The Brainy Insights, a market research company. According to the report, the global pancreatic cancer market is expected to grow at a compound annual growth rate (CAGR) of 8.13% during the forecast period between 2022 to 2030. Rising alcohol and tobacco consumption prevalence expecting to contribute to the rapid growth. As a result, increased emphasis on R&D in molecular biology by private and public players will provide lucrative opportunities. The North American region emerged as the largest market sector, with a 38.5% share of the market revenue in 2021. Emerging targeted therapies, such as multi-targeted T-cell therapy for treating pancreatic cancer, received orphan drug from the US Food and Drug Administration (FDA) early 2022.
By Propanc Biopharma, Inc. · Via Business Wire · July 25, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that a peer reviewed scientific article published by the Company and its research partners reached 3,000 reads on July 6, 2023, according to ResearchGate. The achievement demonstrates reader interest above 93% of published research articles over the past year, “therefore, this shows exceptional interest in our work and bodes well for our future, both clinical and academic,” according to Dr Julian Kenyon, MD, MB, ChB, Propanc’s Chief Scientific Officer.
By Propanc Biopharma, Inc. · Via Business Wire · July 13, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that synthetic recombinant proenzymes trypsinogen and chymotrypsinogen were successfully produced via the Proenzyme Optimization Project 1 (POP1) joint research and drug discovery program with the Universities of Jaén and Granada, Spain. The POP1 project is led by Mr. Aitor González, whose doctoral thesis is focused on the “synthetic development of PRP and its subsequent biological validation,” conducted at the laboratory of Professor Macarena Perán, PhD, University of Jaén, Granada, Spain, and in collaboration with Professor Diethard Mattanovich at the Institute of Microbiology and Microbial Biotechnology, University of Natural Resources and Life Sciences, Vienna, Austria. The program is designed to produce a backup clinical compound to the Company’s lead product candidate, PRP, which is from bovine origin, targeting metastatic cancer from solid tumors. According to Emergen Research, the global metastatic cancer market is projected to be worth over $111 Billion by 2027.
By Propanc Biopharma, Inc. · Via Business Wire · July 5, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced a shareholder update including recent developments and forecast for 2023/24, as Propanc prepares to file a Form 10-K annual report, whose fiscal year end is June 30. The Company is entering into a transformational stage as it prepares for the advancement of its lead product candidate, “PRP,” to enter a Phase Ib, First-In-Human (FIH) study in patients with advanced solid tumors.
By Propanc Biopharma, Inc. · Via Business Wire · June 22, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCBD) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced the Company’s strategic pharma partnering initiative, as its lead product candidate, PRP, advances towards a Phase I, First-In-Human (FIH) study in advanced cancer patients. Over the past several years, management has initiated discussions with potential strategic collaborators to provide the resources to advance PRP into clinical development and for future commercialization. These include Australia’s largest cancer research institute, a merged group of hospitals located in the Andalusian region of Spain and a multi-billion-dollar, global, biomedical company with over 50,000 employees. The strategic goal of these potential collaborations is to develop and commercialize PRP for the treatment and prevention of metastatic cancer from solid tumors in major pharmaceutical markets worldwide.
By Propanc Biopharma, Inc. · Via Business Wire · June 6, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCBD) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company’s intellectual property (IP) portfolio made important advancements in Europe. The dosing patent, describing claims for the dosing regimen of the Company’s lead product candidate, PRP, has now been validated in 12 countries across Europe. In further news, the Company’s method to treat cancer stem cells (CSCs) using PRP received a notice of allowance from the European Patent Office (EPO). This is the third patent allowed, or granted to the Company in Europe, with further applications under examination by the EPO.
By Propanc Biopharma, Inc. · Via Business Wire · May 25, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company’s intellectual property (IP) portfolio achieved significant milestones in North America. The foundation patent, covering composition claims for the Company’s lead product candidate, PRP, received a notice of allowance from the Canadian Intellectual Property Office (CIPO). In further news, the Company’s method to treat cancer stem cells (CSCs) using PRP was granted a patent by the United States Patent and Trademark Office (USPTO). This is the third patent granted to the Company in the US, with further applications under examination by the USPTO.
By Propanc Biopharma, Inc. · Via Business Wire · May 23, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company’s board of directors (the “Board”) has approved a reverse stock split of its common stock at a ratio of 1 post-split share for every 1,000 pre-split shares. The reverse stock split was approved by the Board and the Company’s majority stockholder, representing the majority vote of the stockholders of the Company, on May 1, 2023.
By Propanc Biopharma, Inc. · Via Business Wire · May 22, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that PRP suppresses the TGF-β pathway and the tumor microenvironment in pancreatic cancer, elucidated by one of the Company’s joint researchers, Mrs. Belén Toledo Cutillas MSc, at the laboratory of Professor Macarena Perán, PhD, University of Jaén, Granada, Spain. The experiments were conducted with a well-known small molecule inhibitor of the same pathway, comparing it against the effects of PRP, with results showing an even greater suppression by Propanc’s novel cancer therapy. TGF-β is a growth factor molecule involved in cell proliferation, migration and survival, and death that influences tumor growth in advanced forms of cancer.
By Propanc Biopharma, Inc. · Via Business Wire · March 28, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that PRP exerts significant effects against chemoresistant pancreatic tumor cells, as reported by one of the Company’s joint researchers, Mrs. Belén Toledo Cutillas MSc, at the laboratory of Professor Macarena Perán, PhD, University of Jaén, Granada, Spain.
By Propanc Biopharma, Inc. · Via Business Wire · March 16, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that Mrs. Belén Toledo Cutillas MSc, Joint Researcher, presented at the recent 43rd Meeting of the European Organization of Research and Treatment of Cancer (EORTC), Pharmacology and Molecular Mechanisms (PAMM) group. Mrs. Toledo Cutillas discussed novel approaches to hampering tumor support of key components within the tumor microenvironment (TME). Specifically, she described how “a protein-based treatment re-educates” certain tumor cells that play a key role in the TME, decreasing the TME influence on tumorigenesis and increasing drug uptake of standard therapies that are often rendered ineffective due to chemoresistance. Mrs. Toledo Cutillas commented that results and discussion on the novel approach was well received at the Congress in Florence, Italy.
By Propanc Biopharma, Inc. · Via Business Wire · January 11, 2023

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company’s Joint Researcher, Mrs. Belén Toledo Cutillas, MSc, at the laboratory of Professor Macarena Perán, PhD, University of Jaén, Spain, received an award for best poster at the “Doctoral Days 2022 for Young Researchers of the University of Jaén,” conference. The poster, entitled, “Blocking Tumor Support from Cancer Associated Fibroblasts in Tumor Microenvironment,” describes a novel therapeutic strategy to decrease tumor microenvironment (TME) influence in drug uptake, immune evasion, tumor progression and further tumor dispersion. A copy of the publication can be accessed from the Company’s website, via the following link: https://www.propanc.com/news-media/publications.
By Propanc Biopharma, Inc. · Via Business Wire · December 15, 2022

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that Chief Scientific Officer and Co-Founder, Dr. Julian Kenyon MD, MB, ChB, has recently come to the conclusion that PRP could become an effective chemosensitizer agent against pancreatic cancer. Chemotherapy activates certain growth factors, which directly activate cancer-associated fibroblasts (CAFs) to induce collagen deposits in pancreatic ductal adenocarcinoma, thus increasing tumor resistance and becoming unresponsive to treatment, according to Kim, et al., Nature Communications, journal, October 22, 2022. Pancreatic adenocarcinoma (PDAC) accounts for 80% of pancreatic cancers and has a 5-year survival rate of less than 8%. According to Dr. Kenyon, chemotherapy-induced fibrosis in PDAC highlights an “opportunity for a combinatorial therapeutic strategy to treat these resistant tumors.”
By Propanc Biopharma, Inc. · Via Business Wire · November 30, 2022

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company’s lead product candidate, PRP, could enhance the effects of novel therapies like immune checkpoint inhibitors that can have a role in pancreatic cancer treatment. Chief Scientific Officer and Co-Founder, Dr. Julian Kenyon MD, MB, ChB, predicts therapies can enhance the patient’s immune response to fight solid tumors by enabling detection of specific tumor cells within the body that were previously undetected. For example, once considered a “non-immunogenic” cancer, pancreatic ductal adenocarcinoma (PDA) has been identified with upregulated immune networks and immune checkpoint molecule expression in its tumor microenvironment and is now redefined as an immunogenic cancer, according to the World Journal of Gastroenterology, November 21, 2016.
By Propanc Biopharma, Inc. · Via Business Wire · November 15, 2022

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that Mr. James Nathanielsz, Propanc’s Chief Executive Officer and Co-Founder, will conduct investor meetings and present at the upcoming Sidoti & Company’s upcoming Micro-Cap Virtual Investor Conference, which will be held on Wednesday and Thursday, November 9 – 10, 2022.
By Propanc Biopharma, Inc. · Via Business Wire · October 26, 2022

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that Mr. James Nathanielsz, Propanc’s Chief Executive Officer, will attend the 43rd Meeting of the European Organization of Research and Treatment of Cancer (EORTC), Pharmacology and Molecular Mechanisms (PAMM) group. Professor Perán, Propanc’s Lead Joint Researcher, will also be presenting at the annual EORTC-PAMM meeting to be held in Florence, Italy, December 15 – 17. Topics covered will be innovation and application of pharmacological knowledge to cancer drug discovery and development, with particular regard to innovative and advanced drugs and their mechanisms of action, pharmacometrics and clinical application in oncology. Professor Perán, supported by Mrs. Belén Toledo Cutillas MSc and Mr. Aitor González from Professor Perán’s laboratory at Jaén University will present findings on the anti-cancer effects of the Company’s lead product candidate, PRP, effects on the tumor microenvironment and upscaling of synthetic recombinant protein production for a backup clinical compound for PRP.
By Propanc Biopharma, Inc. · Via Business Wire · October 12, 2022

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that Chief Scientific Officer and Co-Founder, Dr Julian Kenyon, MD, MB, ChB, explains why pancreatic and ovarian cancers are selected as the primary target therapeutic indications for planned PRP human studies. According to Dr Kenyon, target indications were selected based on in vitro and in vivo data, as well as clinical observations from a compassionate use study investigating the effects of two proenzymes, trypsinogen and chymotrypsinogen against a range of malignant tumors. Overall, proenzymes appeared to exert significant effects against more aggressive, less differentiated tumor types, like pancreatic and ovarian tumors. Patients from the compassionate use study suffering from cancers of the GI tract, or endocrine tumors, such as pancreatic and ovarian cancers, benefited most from treatment. The world market for pancreatic and ovarian cancer drugs is projected to grow to $4.2 Billion in 2025 according to Grandview Research and $10.1 Billion by 2027 according to iHealthcareAnalyst, respectively, resulting in a combined global market of $14.3 Billion over the next 5-year period.
By Propanc Biopharma, Inc. · Via Business Wire · September 29, 2022

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced a shareholder update including recent developments and forecast for the remainder of 2022 and 2023, as the Company prepares to file a 10K annual report which is due on September 30. The Company’s main focus of operations is preparing its lead product candidate, PRP, to enter clinical development for the treatment and prevention of metastatic cancer. PRP represents a novel therapeutic approach, targeting and eradicating cancer stem cells, but leaving healthy stem cells alone, making it less toxic compared to current standard treatment options, like chemotherapy and radiotherapy. The Company also has a joint research and drug discovery program, “POP1,” (Proenzyme Optimization Project 1) which is designed to produce a fully synthetic recombinant back up clinical compound to PRP, which is naturally derived. A second joint research program is also underway investigating future possible therapeutic applications of PRP in a clinical setting.
By Propanc Biopharma, Inc. · Via Business Wire · September 13, 2022

Propanc Biopharma, Inc. (OTC Pink: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced significant effects of PRP against the tumor microenvironment and pre-metastatic niche has been reported by the Company’s joint researcher, Mrs. Belén Toledo Cutillas MSc, at the laboratory of Professor Macarena Perán, PhD, University of Jaén. Treatment with PRP was shown to have a favorable impact inhibiting, slowing, or reversing tumor development by acting as an anti-tumor agent, decreasing tumor cell proliferation, developing a non-malignant phenotype (observable characteristics) and promoting cell adhesion (sticking close to one another) and differentiation (cell specialization rather than stem cell like). It was concluded that PRP could have a significant impact on the tumor microenvironment as a potential clinical application. PRP is a combination of the two proenzymes trypsinogen and chymotrypsinogen.
By Propanc Biopharma, Inc. · Via Business Wire · September 8, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that recombinant trypsinogen and chmyotrypsinogen were synthesized via the Proenzyme Optimization Project (POP1) joint research and drug discovery program. In the case of trypsinogen, the initial success of producing trypsinogen synthetically has now advanced to the stage where optimization of protein production is underway, whereas purification and yield of chymotrypsinogen is currently the focus of research. The program is designed to produce a backup clinical compound to the Company’s lead product candidate, PRP, which is targeting metastatic cancer from solid tumors. According to Emergen Research, the global metastatic cancer market is projected to be worth $111 Billion by 2027.
By Propanc Biopharma, Inc. · Via Business Wire · August 23, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that a Notice of Allowance has been received from the European Patent Office (EPO) for claims involving compositions of proenzymes to treat cancer. This is the Company’s second patent application allowed in this jurisdiction and expires in November, 2036. A third patent application is currently under examination at the EPO for a method to treat cancer stem cells, which was allowed in March this year by the US Patent and Trademark Office (USPTO).
By Propanc Biopharma, Inc. · Via Business Wire · August 16, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that a Joint Research Collaboration Agreement has been established with the Universities of Jaén and Granada, Spain. Since late 2020, Mrs. Belén Toledo Cutillas MSc, has been investigating an important experimental thesis on the effects of proenzyme therapy and the impact on the tumor microenvironment, which is key to the development, invasion, metastatic spread and recurrence of solid tumors. The work is being conducted at the laboratory of Professor Macarena Perán PhD, who is the lead researcher on the project and is the second Joint Research Collaboration Agreement currently in progress with the two Spanish Universities.
By Propanc Biopharma, Inc. · Via Business Wire · August 3, 2022
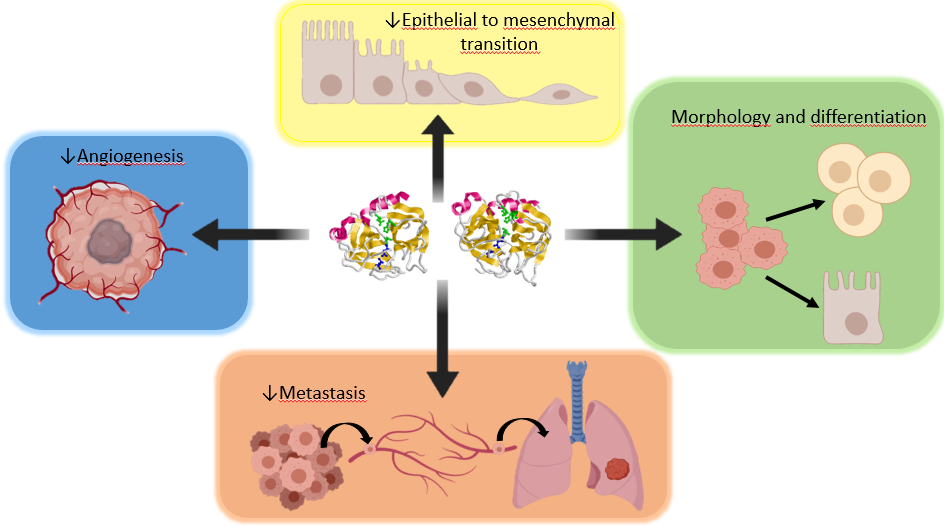
Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that Chief Scientific Officer and Co-Founder, Dr Julian Kenyon, MD, MB, ChB, reflects on the unique anti-cancer effects of PRP discovered as a result of the significant and diligent research invested by the Company and its joint research team over the past decade. PRP is a proenzyme therapy for the treatment and prevention of metastatic cancer from solid tumors. This unique approach could become an effective tool in the fight against metastatic cancer, which is the main cause of patient death for sufferers. PRP is considered unique because rather than kill cancer cells like most standard therapies, proenzymes induce cancer cells to differentiate so they are no longer malignant and die off naturally, “thus preventing these dangerous cells to spread and metastasize,” according to Dr Kenyon.
By Propanc Biopharma, Inc. · Via Business Wire · July 28, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that successful production of a synthetic recombinant version of the proenzyme trypsinogen was completed via the Proenzyme Optimization Project 1 (POP1) joint research and drug discovery program. The program is designed to produce a backup clinical compound to the Company’s lead product candidate, PRP, which is targeting metastatic cancer from solid tumors. According to Emergen Research, the global metastatic cancer market is projected to be worth $111 Billion by 2027.
By Propanc Biopharma, Inc. · Via Business Wire · July 19, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that CEO and Co-Founder Mr. James Nathanielsz, BAS, MEI, believes the Company’s lead asset could unlock value as PRP advances to a Phase I, First-In-Human study in advanced cancer patients. As a less toxic therapy compared to standard treatments with a unique approach for the treatment and prevention of metastatic cancer, PRP has the potential to be a welcome addition to the treatment process that is complementary to existing therapies. Notwithstanding recent advances in the oncology sector, metastasis from solid tumors remains the unsolved final frontier and is the biggest killer of cancer sufferers.
By Propanc Biopharma, Inc. · Via Business Wire · July 12, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that CEO and Co-Founder, Mr. James Nathanielsz, BAS, MEI, expresses confidence over the Company’s growing intellectual property portfolio. Presently, there are 39 granted patents and a further 26 patent applications under examination in key global jurisdictions relating to proenzymes as an effective therapeutic agent against solid tumors, covering the lead product candidate, PRP.
By Propanc Biopharma, Inc. · Via Business Wire · July 5, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company filed a Definitive Information Statement under Section 14C of the Exchange Act on June 9, 2022, to increase the authorized shares of common stock and the option to effectuate a reverse split of common stock at the discretion of the Board. Mr. James Nathanielsz, BAS, MEI, CEO and Chairman at Propanc, believes the increase in authorized shares is necessary for the Company as a result of recent volatile market conditions, which have been broad and far reaching for microcap companies. Regarding the option for a reverse split of common stock, Mr. Nathanielsz does not foresee the Board proceeding in the near future due to a number of factors.
By Propanc Biopharma, Inc. · Via Business Wire · June 24, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the results from a small trial of just 18 rectal cancer patients in complete remission using an immunotherapy called dostarlimab are “impressive,” whilst acknowledging there’s more work to be done. Chief Scientific Officer and Co-Founder, Dr Julian Kenyon MD, MB, ChB, believes that the field’s biggest challenge remains that immunotherapies work inconsistently across cancers. Oncologists estimate a response rate of 20% across cancer types, according to the Wall Street Journal (“WSJ”). The drugs can wipe out cancers from some people, but fail to work for others. It is also uncertain whether the cancer may eventually return once a patient is in remission, even after a prolonged period of time.
By Propanc Biopharma, Inc. · Via Business Wire · June 22, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company’s lead product candidate, PRP, targets solid tumors, which accounts for 80 – 90% of cancer cases, according to the National Cancer Institute. Chief Scientific Officer and Co-Founder, Dr Julian Kenyon MD, MB, ChB, is leading research into a novel approach to prevent recurrence and metastasis from solid tumors using pancreatic proenzymes that target and eradicate cancer stem cells (CSCs), which are a small subpopulation of cells within tumors capable of self-renewal, differentiation and tumorigenicity when transplanted into an animal host. CSCs is the mechanism by which cancer is able to return and spread, even post standard treatments.
By Propanc Biopharma, Inc. · Via Business Wire · June 14, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that Chief Scientific Officer and Co-Founder, Dr Julian Kenyon MD, MB, ChB, believes the reduced toxicity of PRP compared to standard treatment approaches will impact cancer patient lives significantly. Many standard therapies for advanced cancer urgently need improvement, generally providing modest benefits and frequently causing adverse effects. Propanc’s focus is to provide oncologists and their patients with therapies for metastatic cancer which are more effective than current therapies and have a substantially reduced side effect profile. According to Cancer Treatment Centers of America, for all the advances made in cancer treatment over the past several decades, one statistic has remained unchanged: Metastatic cancer accounts for up to 90% of all cancer deaths in the United States each year.
By Propanc Biopharma, Inc. · Via Business Wire · June 1, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that 100 years of clinical evidence supporting the use of proenzymes as a new therapeutic approach to treat cancer can be considered ‘compelling’. Chief Scientific Officer and Co-Founder, Dr Julian Kenyon MD, MB, ChB, has researched the effects of proenzymes against cancer for over 15 years and first came across the technology in his search to extend the life of several late-stage patients suffering from malignant solid tumors in the mid 2000’s. It was Professor John Beard from Edinburgh University who first proposed that pancreatic enzymes represent the body’s primary defense against cancer and would be useful as a cancer treatment. Since then, several scientists have endorsed Beard’s hypothesis with encouraging data from patient treatment.
By Propanc Biopharma, Inc. · Via Business Wire · May 26, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the Company is undertaking manufacturing and development of PRP for human use. PRP is the Company’s lead product candidate and is a novel pharmaceutical formulation consisting of two proenzymes to be administered by I.V. injection in a world first, First-In-Human (FIH) study in advanced cancer patients. It is a long-term therapy for the treatment and prevention of metastatic cancer in patients suffering from solid tumors.
By Propanc Biopharma, Inc. · Via Business Wire · May 18, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced a novel way to stop cancer by inducing a process called “cell differentiation”. Known as differentiation therapy, the key objective is to convince malignant cells to stop proliferating and return to do their work as a specific cell type. There are advantages of differentiation therapy over conventional therapeutic strategies. Differentiation therapy does not target cell death, so healthy cells within the patient will not be compromised, unlike chemotherapeutic drugs or gamma irradiation.
By Propanc Biopharma, Inc. · Via Business Wire · May 11, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that pharma grade raw materials were purchased for the manufacture of PRP in preparation for the Phase I First-In-Human (FIH) study in advanced cancer patients suffering from solid tumors. Approximately 0.5kg of trypsinogen and 2.4kg of chymtrypsinogen was procured initially, with a second half of the same batch quantities to be purchased towards the middle of this year. The total amount of raw materials purchased is expected to be sufficient for the early-stage clinical development plan for PRP, which is administered by intravenous (I.V.) injection, once weekly. The first FIH study is planned for treatment of up to 30 to 40 patients with advanced solid tumors. This will be followed by up to two 60 patient Phase II studies in patients suffering from pancreatic and ovarian tumors.
By Propanc Biopharma, Inc. · Via Business Wire · May 2, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced why the Company’s management team believes that cancer patients are likely to respond to PRP treatment. PRP is the Company’s lead product candidate for the treatment and prevention of metastatic cancer from solid tumors, which is the main cause of death for sufferers. PRP is currently advancing towards a Phase I, First-In-Human (FIH) study, in advanced cancer patients. PRP is a mixture of two proenzymes, trypsinogen and chymotrypsinogen from bovine pancreas administered by intravenous injection. A synergistic ratio of 1:6 inhibits growth of most tumor cells. Examples include kidney, ovarian, breast, brain, prostate, colorectal, lung liver, uterine and skin cancers.
By Propanc Biopharma, Inc. · Via Business Wire · April 13, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that its Chief Executive Officer, Mr. James Nathanielsz, expressed concern over disruption to cancer care during the global pandemic, where patients are experiencing delays to diagnoses and treatment, to halting clinical trials. An increased risk of infection as a result of the pandemic is also a real and significant threat to the lives of cancer sufferers whilst undergoing treatment. Mr. Nathanielsz emphasized a pressing need for new and less toxic treatments must be a high priority, especially approaches that do not suppress the immune response of the patient.
By Propanc Biopharma, Inc. · Via Business Wire · April 6, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that a Notice of Allowance has been received from the US Patent and Trademark Office (USPTO) for claims involving a novel method to treat cancer stem cells (CSC’s). The allowed US patent protects proprietary claims capturing methods and uses for pancreatic proenzymes to treat cancer by specifically targeting and eradicating CSCs.
By Propanc Biopharma, Inc. · Via Business Wire · March 22, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that its Chief Executive Officer, Mr. James Nathanielsz, believes the Company’s proenzyme therapy may become the healthcare solution of choice for treating cancerous solid tumors. As a result of remarks made by President Biden recently, the White House is determined to help millions of Americans protect and preserve their health, all by making the cost of prescription drugs much more reasonable. Under the “Build Back Better Bill” they plan to end the days when drug companies could increase their prices with no oversight and no accountability. Outrageous costs, affecting everyone across the board, spanning every kind of condition and disease. Mr. Nathanielsz agrees this approach to innovation by the pharma/biotech sector is long overdue and no longer can they set the drug price at whatever the market will bear. The sector must continue to find ways to innovate with new and improved drugs, but not at great expense to the patient. No longer can America afford to subsidize the rest of the world in healthcare.
By Propanc Biopharma, Inc. · Via Business Wire · March 16, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that the joint research partnership with the Universities of Jaén and Granada, Spain, is entering its thirteenth year since their collaboration began in 2009. The research partnership is led by Professor Macarena Perán, Ph.D., from Jaén University, supported by Professor Juan Marchal M.D., Ph.D., at the University of Granada, and Dr. Julian Kenyon M.B.Ch.B., M.D., Chief Scientific Officer at Propanc Biopharma. The enduring partnership with the Universities, as well as working with contract research organizations (CRO’s) and contract manufacturing organizations (CMO’s) has led to the Company’s lead product candidate, PRP, advance towards a First-In-Human clinical study and plans to develop a backup clinical candidate from the POP1 joint research and drug discovery program also underway.
By Propanc Biopharma, Inc. · Via Business Wire · February 23, 2022

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced that Ms. Belen Toledo MSc., from the laboratory of Professor Macarena Perán Ph.D., at the University of Jaén, Spain, recently completed an important experimental thesis on the effects of proenzyme therapy and the impact on the tumor microenvironment, which is key to the development, invasion, metastatic spread, and recurrence of solid tumors. Ms. Toledo also reconfirmed proenzymes kill cancer stem cells (CSCs). This research is part of the “Proenzymes Optimization Project 1” (POP1) Joint Research and Drug Discovery Program at the Universities of Jaén and Granada, Spain, designed to produce synthetic recombinant, commercial scale quantities of the two proenzymes, trypsinogen and chymotrypsinogen.
By Propanc Biopharma, Inc. · Via Business Wire · December 21, 2021

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced the entering into an equity purchase facility of up to $5 million with Dutchess Capital Growth Fund LP (“Dutchess”). Funds raised will be used to support operations as management advances the Company’s lead product, PRP, to a First-In-Human study in advanced cancer patients suffering from solid tumors. Founded in 1996, Dutchess and its managed investment funds have provided principal-based financing and advisory services for publicly-traded and pre-IPO growth companies worldwide, partnering with over 250 micro- and small-cap companies.
By Propanc Biopharma, Inc. · Via Business Wire · December 14, 2021

Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, today announced the appointment of Paul Patrizio as Strategic Advisor to the Propanc Executive team. Mr. Patrizio will report directly to the Company’s Chief Executive Officer, James Nathanielsz. The Company has also initiated the process of establishing a wholly owned US based, R&D operating subsidiary, Cellmed Bio LLC in New Jersey, one of the largest biotech hubs in the country.
By Propanc Biopharma, Inc. · Via Business Wire · November 3, 2021
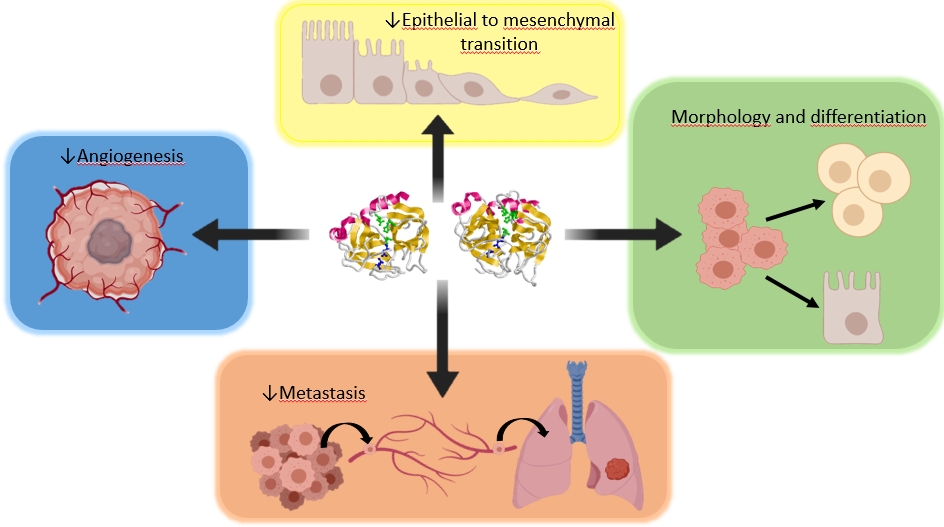
Propanc Biopharma, Inc. (OTCQB: PPCB) (“Propanc” or the “Company”), a biopharmaceutical company developing novel cancer treatments for patients suffering from recurring and metastatic cancer, announced today that the Company’s scientific researchers, together with its joint research partners, Universities of Jaén and Granada, published key data in a peer reviewed journal, Expert Opinion on Biological Therapy, confirming the anti-tumor potential of a mixture of two pancreatic proenzymes trypsinogen and chymotrypsinogen. Treatment with proenzymes sensitizes cancer stem cells (CSCs) which may allow standard treatment approaches, like chemotherapy and radiotherapy to be more effective. Expert Opinion on Biological Therapy is a peer-reviewed medical journal covering research on all aspects of biological therapies. The article is entitled “Trypinsogen and chymotrypsinogen: potent anti-tumor agents,” and can be accessed via the link: https://www.tandfonline.com/eprint/MJ7Y7EWCJI9CHESREZUC/full?target=10.1080/14712598.2021.1922666
By Propanc Biopharma, Inc. · Via Business Wire · May 17, 2021
